Beyond Cau's riddle
The title and entire post refer to this year’s publication by Andrea Cau and Alessandro Paterna: Beyond Stromer’s Riddle: the impact of lumping and splitting hypotheses on the systematics of the giant predatory dinosaurs from northern Africa. After reviewing the paper, I find it ironic that there is a clear contradiction between the authors' declared approach and their actual practices.
One of the main messages conveyed in the paper is a criticism of aprioristic lumping or splitting - that is, applying either approach rigidly to all cases without assessing the specific evidence. The authors argue that both lumping and splitting can lead to errors if used dogmatically. However, they also clearly state that in the absence of direct overlap of skeletal material, methodological splitting should be the default hypothesis: isolated bones should be treated as separate OTUs in phylogenetic analyses, because this reduces the risk of introducing artificial, chimerical taxa. This approach aims to avoid lumping unrelated specimens based only on assumed similarities or stratigraphic proximity.
Contrary to this declared stance, however, are the actions taken with respect to Bahariasaurus ingens and Deltadromeus agilis, which only superficially meet the requirements of justified lumping. Cau identifies IPHG 1912 VIII 82 (originally described by Stromer as the pubis of an indeterminate theropod) as the ischium of Bahariasaurus, and designates it as a “Rosetta stone” - a key link connecting Bahariasaurus with Deltadromeus. However, in the case of Deltadromeus, the preserved portion of the ischium includes only the distal fragment. So, despite some clear similarities, the amount of comparable material is very limited, which automatically calls the validity of this action into question.
More problematic, however, is the fact that distinct anatomical differences visible in the remaining overlapping material (i.e., middle caudal vertebra and scapulocoracoid) were ignored. These differences undermine the justification for lumping the taxa, and yet the authors proceeded to assign to Bahariasaurus additional material from the Bahariya Formation that does not overlap in any way with the Bahariasaurus type or referred material but can only be meaningfully compared with Deltadromeus (e.g., the femur IPHG 1912 VIII 69, fibula IPHG 1912 VIII 70, and humerus IPHG 1912 VIII 177). Such a practice directly contradicts the supposedly preferred option of splitting when overlap is lacking.
Another clear and main contradiction of the declared methodology is the coding of all this material (Bahariasaurus + Deltadromeus + additional Bahariya material) as a single taxon (OTU) in the phylogenetic analysis, without testing the alternative hypothesis of distinctiveness - which would have been more appropriate under the authors’ own recommended approach of methodological splitting. It would have been reasonable to check whether Bahariasaurus and Deltadromeus, when coded as separate OTUs, turn out to be closely related phylogenetically or not.
In the following post, I will discuss in detail the shortcomings of the methodology employed by the authors, critique the proposed synonymy of Bahariasaurus ingens and Deltadromeus agilis, review the nature and taxonomic status of Bahariasaurus ingens, and comment on some other discoveries described by Stromer.
Synonymy of Bahariasaurus and Deltadromeus
The issue of the synonymy of these two taxa was treated rather superficially in the paper by Cau and Paterna; a broader - but still poor - perspective on the situation is provided by Cau’s later blog posts. He wrote some posts on his Facebook page, referring to intraspecific variation in Tyrannosaurus rex, comparing and listing features, Elaphrosaurus, Bahariasaurus, the Rosetta Stone… he even decided to explain to us why Deltadromeus is not a tetanuran and not a synonym of Neovenator. All of this helps to overlook the real clue, which is that the overlapping material between Deltadromeus and Bahariasaurus (the “Rosetta Stone”) within the ischium consists only of the distal fragment (apparently there exists a proximal portion of the ischium, but for some reason it wasn’t used). Which would be potentially helpful since Ibrahim et al, 2020 stated that Bahariasaurus differs from Deltadromeus ,,most noticeably in the shape of the iliac peduncle of the ischium’ which likely refers to the proportionally narrower iliac peduncle in Bahariasaurus (Sereno et al., 1996). In reality, Bahariasaurus shares certain features with Deltadromeus in the distal portion of the ischium, yet none of these appear to be unique. The main character shared by both is an anteroposteriorly expanded ischial boot, a condition also present in Elaphrosaurus, Masiakasaurus, Neovenator, Concavenator, Alpkarakush, and Megalosaurus. Although it is worth noting that the degree and angle of expansion most closely resembles that seen in the mentioned abelisauroids.
However, this is where the clear similarities end. The slenderness of the shaft is not particularly informative or diagnostic - especially given that the ischium of Bahariasaurus represents a juvenile morphology. It is worth pointing out that the clearly smaller Bahariasaurus possesses a more robust shaft than the larger, more mature Deltadromeus. Another similarity is the narrow boot in distal view; however, contrary to Cau, this condition does not differ in any significant way from what is seen in Neovenator, and is also present in other theropods. Bahariasaurus differs from Deltadromeus in having an unfused ischial boot posteriorly, though this may be an ontogenetic trait.
Edit (20/0725/ when scaled to the same shaft width, Deltadromeus (right) shows much more posteriorly expanded ischial boot
In summary, Bahariasaurus does not share any unique, species-level features with Deltadromeus, and the observed similarity appears negligible when compared to the clear differences evident in other overlapping material between Bahariasaurus and Deltadromeus (such as middle caudal vertebrae and the scapulocoracoid). Taking all this into account, the distal morphology of the ischium in Bahariasaurus can at best be considered a factor suggesting abelisauroid affinities, and going beyond that towards synonymization is like cherry-picking a few details in order to push this biased assumption ignoring anatomical premises.
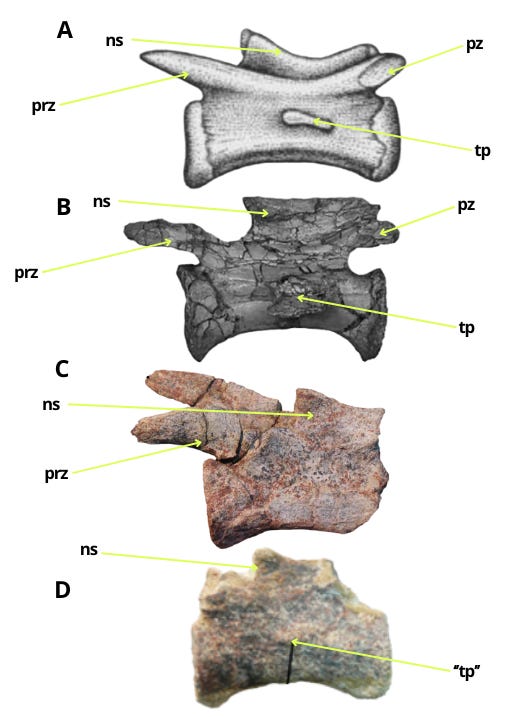
The middle caudal vertebrae of Bahariasaurus and Deltadromeus differ significantly, as shown in Figure 1. The Egyptian taxon exhibits neural spines in the form of a weakly developed longitudinal crest, in contrast to Deltadromeus, which possesses a distinct and well-defined neural spine (1). Furthermore, Bahariasaurus displays less prominent prezygapophyses and more pronounced postzygapophyses, the opposite condition to that observed in Deltadromeus (2). Finally, the transverse process in Bahariasaurus is positioned at the boundary between the centrum and the neural arch, whereas in Deltadromeus, it is reduced and located at mid-height of the centrum (3). The author is aware that IPHG1912VIII83 had completely preserved prezygapophyses ;)
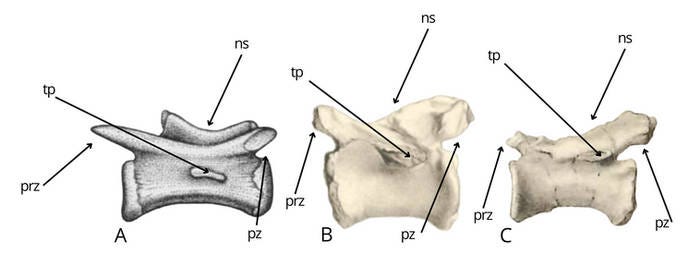
Of course, an attempt was made to explain this by invoking positional variation along the vertebral column. However, such a proposed pattern would be inconsistent with the condition observed in theropods. Deltadromeus exhibits a reduced transverse process, while retaining a well-developed neural spine. It is not plausible that the vertebrae of Bahariasaurus, if they were more distal (when a reduced neural spines takes priority), would instead exhibit a more developed transverse process. Nor is it plausible that, if they were more proximal (when a more developed transverse process takes priority), they would show a less developed neural spine. It is worth noting that we had a distal caudal vertebra of Bahariasaurus (with a completely reduced transverse process). Although its incompleteness makes direct comparison difficult, it most likely corresponds in morphology to the middle caudals, and therefore clearly differs from the vertebrae of Deltadromeus, undermining the possibility of explaining these differences by positional variation along the vertebral column. While this is not entirely certain, it is significant that Stromer, in his diagnosis of the taxon, when referring to this posterior caudal, wrote: “Neural roof low, with edge as proc. spin.; prezygapophyses short, rising up,” which strongly suggests that the lack of a distinct, tall neural spine (as seen in Deltadromeus) and the absence of long, well-developed prezygapophyses (as in Deltadromeus) are not due to incompleteness but reflect the actual morphology of the vertebra and taxon.
These are clear-cut differences between caudal vertebrae that cannot be explained by individual variation, ontogeny, positional differences, or whatever..
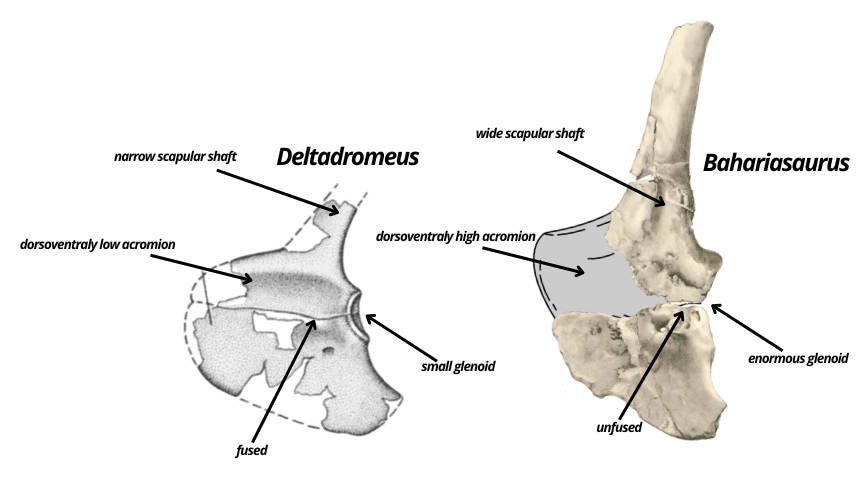
Another overlapping element that reveals the distinctiveness of both taxa is the scapulocoracoid. Bahariasaurus differs clearly in having a non anteroposteriorly expanded coracoid, a broader scapular shaft, concave scapular margin and a distinctly convex coracoid at the junction with the scapular surface. Furthermore, the acromion - although not preserved - can be reasonably reconstructed based on the articulation surfaces of the preserved fragments. This reconstruction clearly indicates that Bahariasaurus had a much taller acromion in dorsoventral height. Another clear difference concerns the fusion of the scapula and coracoid. Deltadromeus shows a completely fused scapulocoracoid, whereas in Bahariasaurus these elements seem to be unfused. This is highly unexpected if Deltadromeus were a younger individual of Bahariasaurus, as older individuals should exhibit more extensively fused elements. Another key difference lies in the size of the glenoid. Basal abelisauroids, including Deltadromeus, are characterized by slender, relatively non-functional forelimbs, which is evident in the small glenoid and slender humeri with a small humeral head. In contrast, Bahariasaurus possesses an enormous glenoid, which - combined with the large surface area of the acromion and pronounced probable muscle attachment scars - suggests a robust, heavily muscled humerus. Interestingly, this was already noted by Stromer in his description of IPHG 1912 VIII 177 (the humerus that Cau and Paterna assigned to Bahariasaurus), when he explicitly rejected the possibility that this humerus belonged to Bahariasaurus, stating that “because they (pectoral girdle elements) do not fit to a reduced forelimb’’.
An important point is that the anterior caudal vertebrae of IPHG 1912 VIII 60, based on Stromer's measurements, are 12–13 cm in length. This is noteworthy given that the anterior caudal vertebrae of Deltadromeus, measured from Sereno (1996), also have a neural arch length of approximately 12-13 cm at the base. This indicates that both animals possessed anterior caudals of comparable size - an element that is ontogenetically isometric. This implies that if both specimens were to represent the same species, then - given equal caudal size - they should also be very similar in overall size and morphology. In reality, although both elements are of comparable size, their morphology is significantly different, as demonstrated above and below. This comparison further highlights the differences, clearly indicating that these two taxa are distinct.
Edit (20/07/25) I’ve just found that the middle and distal caudal vertebrae of Deltadromeus have centrum lengths of 13 cm (Sereno et al. 1996), which means they are actually larger than the distal caudal vertebrae of Bahariasaurus, which measure 12 and 12.5 cm. This further supports the idea that, if both specimens belonged to the same species, they should be roughly the same size, based on caudal vertebra size.
However, as has been demonstrated, the morphology of the pectoral girdle is radically different between the two – for example, the glenoid and acromion in Bahariasaurus are more than twice as tall as those same structures in Deltadromeus. Such a difference is impossible between two individuals of the same species, especially if they are of similar body size.
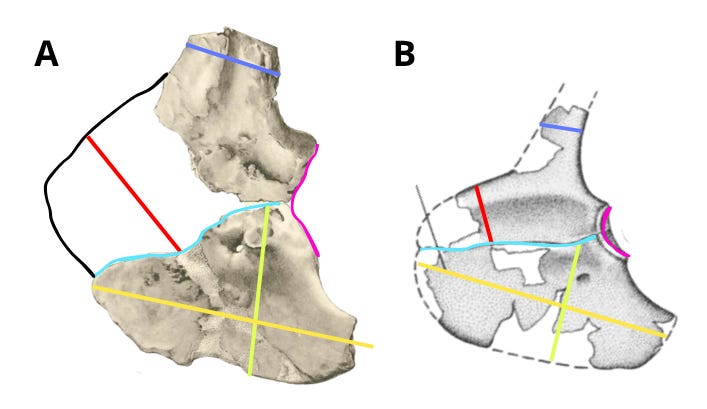
In summary, the comparison of the middle caudal vertebrae and pectoral girdle elements between Bahariasaurus and Deltadromeus reveals a series of clear differences that are highly unlikely - if not outright impossible - to explain by ontogeny, individual variation, etc.
Taxonomic status of Bahariasaurus ingens
Another issue, which has existed forever but has become even more problematic since the latest publication, is the status of Bahariasaurus itself.
This issue has been a subject of debate since the time of its original description, but Cau’s recent publications (2024 and 2025) have added some color to the discussion. In reality, however, they amount to little more than unnecessary confusion, without many truly noteworthy new points.
My broader point is that, contrary to many earlier but rather informal suggestions, Bahariasaurus ingens is not only not a synonym of Deltadromeus agilis, but also does not appear to be closely related to it. This is because the association of this Egyptian taxon with Abelisauroidea is poorly supported - both in phylogenetic analyses and in direct morphological assessment, given the presence of traits that are highly unexpected within that clade.
Cau’s proposal to synonymize it has certain consequences that make the proposed concept of Bahariasaurus ingens inconsistent with actual evidence. Let's start with the fact that Cau’s approach to this Egyptian taxon changed rather quickly. In 2024, he took a very conservative stance, coding only a single specimen (IPHG 1922 X 47), while suggesting that specimens IPHG 1922 X 48 and IPHG 1912 VIII 62 might belong to a different animal. It seems that such a conservative approach is not appropriate. In reality, Stromer listed X47 and X48 as type material, emphasizing their strong morphological similarity, which seems reasonably supported by the available descriptions.
I mention this because Cau’s approach changed significantly over just a few months. In his latest work this year, the taxon Bahariasaurus ingens not only includes the syntypes and paratypes (assigned based on overlapping, comparable material), but also the holotype of Deltadromeus agilis, along with the femur and fibula from Bahariya (IPHG 1912 VIII 69 and IPHG 1912 VIII 70, respectively) and quite random humerus (IPHG 1912 VIII 177), which is not associated with any other material and its referral is based on some similarities to humerus of Deltadromeus.
This approach is also reflected in the character coding, and in turn in the resulting phylogenetic position of the taxon. I want to stress that the recovery of Bahariasaurus within Abelisauroidea in the 2024 and 2025 analyses is simply worthless. In the 2024 analysis, only one specimen was coded, for the reasons described above, which clearly undermines the robustness of the results. The situation is even more interesting in the newest analysis from this year. Bahariasaurus ingens is coded not only based on the true Bahariasaurus material but also the holotype of Deltadromeus, the femur (IPHG 1912 VIII 69), fibula (IPHG 1912 VIII 70), humerus (IPHG 1912 VIII 177), and single characters from the radius and parietal - bones not actually present in the fossil material. All these characters coded from this material significantly affect the analysis outcome, and since none of them (except the true Bahariasaurus material) should have been assigned and coded, the result has no value.
By "true Bahariasaurus ingens material", I refer to the syntypes designated by Stromer, as well as referred material that rest on overlapping, comparable fossil material. Specifically:
IPHG 1922 X 47
IPHG 1922 X 48
IPHG 1922 X 48 B
IPHG 1911 XII 23
IPHG 1912 VIII 62
IPHG 1912 VIII 74
IPHG 1912 VIII 83
IPHG 1912 VIII 60
IPHG 1912 VIII 81?
IPHG 1912 VIII 82
IPHG 1912 VIII 77?
These (apart from these marked by ‘?’) are the specimens upon which Bahariasaurus ingens should be coded to obtain any credible phylogenetic position and no other specimens [Deltadromeus holotype, femur (IPHG 1912 VIII 69), fibula (IPHG 1912 VIII 70) or humerus (IPHG 1912 VIII 177)] should be assigned to this taxon. Obviously, therefore, certain corrections to Cau and Paterna’s version must be implemented. Starting by removing (I mean marking as '?') dozens of characters in Bahariasaurus related to the femur, fibula, humerus, radius, parietal - and, critically, separating out Deltadromeus agilis and deleting the characters that were coded to it based on juvenile Bahariasaurus material. Then one should revise certain characters that, in my opinion, were coded incorrectly. They concern the following characters of Bahariasaurus and other taxa, with main focus on megaraptorans and basal abelisauroids. (the numbering corresponds to the character list used in both the 2024 and 2025 analyses).
Bahariasaurus (Stromer, 1934)
215-? 230-0 236-? 249-? 250-? 256-1 259-0 266-0 270-0 343-? 345-0 351-0 353-0 365-0 384-0 397-0 401-0 407-0 412 - 0 413 - 0 416 - 1 419-1 425-0 427-1 517-0 604-0 629-0 665-0 691-0 748-? 1083-? 1098-? 1135-1 1185-? 1209-1 1349-? 1486-? 1501-0 1502-0 1512-0 1576-? 1643-? 1760-0 1808-? 1863-? 1932-? 1940-?
215 – Presence of this lamina varies among available vertebrae; cannot be safely determined –
?230 – Centra are approximately as tall as they are wide –
0236 – Cannot be determined due to incomplete neural spines –
?249 – Cannot be determined due to incomplete neural spines –
?250 – Cannot be determined due to incomplete neural spines –
?256 – The acromion was likely very expanded –
1259 – Glenoid contribution from scapula and coracoid is roughly equal –
0266 – Mostly flat –
0270 – Can be interpreted as slightly convex –
0343 – No rib preserved on the third sacral –
?345 – No such keel present –
0351 – State based on measurements (note: measurements refer to anterior articular surfaces) –
0353 – Middle neural spines are preserved –
0365 – According to the description, there is no keel and no strong constriction –
0384 – According to the description, strongly flattened –
0397 – Can be stated based on the description –
0401 – Can be stated based on the description –
0407 – Pubic shaft is straight –
0412 – Clearly no distinct perforation –
0413 – No perforations –
0416 – A fenestra is present –
1419 – Can be measured after scaling between specimens –
1425 – Ischium is distally compressed –
0427 – Distal expansion is present –
1517 – No such extension present –
0604 – No lateral process on the pubis –
0629 – Prezygapophyses were short –
0665 – Neural spines are very low –
0691 – Measurable –
0748 – Cannot be stated; middle neural arches are missing –
?1083 – Cannot be stated due to posteriorly incomplete centrum –
?1098 – Cannot be stated with certainty –
?1135 – Measurable –
11185 – Cannot be assessed as apneumatic due to lack of anteriormost caudal neural arches –
?1209 – Proximally expanded –
11349 – Cannot be determined from available illustrations and description –
?1486 – Presence of such fossa is unclear –
?1501 – Zygapophyses project subhorizontally –
01502 – Based on measurements –
01512 – Prezygapophyses are not forked –
01576 – Cannot be determined due to missing anteriormost caudal neural arches –
?1643 – Presence of such fossa is unclear –
?1760 – No such "neck" present –
01808 – Presence of such ridge is unclear –
?1863 – Cannot be determined due to incomplete neural spines –
?1932 – Neural spine is incomplete –
?1940 – Cannot be determined due to incomplete anterior caudal neural spines –
?
?????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????1?????????????0?0??00???0???0?1??0??0?00???0??????0????????10?1??010????01??0?????????????????????????????????????????????????????????????????????????1001???0??????1?0?1000??????1?0?0???????0????????????0???0?????011???0010?010000?0010????????????????????????????????????????????????????????????????????????????????????????0???????????0????????????0?1???????0????0??????????????????????????????????????????????0???????????????????0?11?00?????????????????0???????????0????0??????????????????0??????0??0???????????0????????????????????0???????????????????0???????????11??10??????????1??0??????????????????0??????????1????????????????????????0????1????????01??0???????????????00??????????????????????1??????0???????0???????????0??????0?01??????????????1????????????????????????????????????????????????????????????????0??????????????????????1??0???????0???????????????????????????????0???????????1????????????????0?????????????0???0????????0????1???0?????????????????????0????????1??????????????????????????????????????1??????0???????????1????1??????????????????0??????????????????????????0?????0??0???????0????????????????????????????????????????1?????????0??11?????????????????????????????????????????0?????????????????0????0???????0?????????????0?????0????????0???????????????????????????????????????????????000???????00????????????00????????????????0??????0???1?????????????????????????????0????????????????????????????????????????????????????1??????0?????001????????????????????????????????????????????????0?????????????????0??????0??????????????0??????????????????0???????0?1????????????????0???????????????????????????????????????????????????????1???????????0?????????????????0??????0????????????????????????0???????????????????????????????????????
Elaphrosaurus (Rauhut & Carrano, 2016)
412 – ? 351 – 0 1173 - 0 1174 - 0 1605 – 0
?????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????001?????????000110000000100?1100010?10000110100??100?0?000?10010?1100?0??1???00000??10011100??????????1???????1?????1???????00???1?????0?????????????1?0000?10?00?0?110?00000010?001?0111111010110010110100000100101001????00??0010?0101010010001011100100??11100010010000011??01?00??11??0?110000100001?001??0????1?00????????????0???1?????0??0????????0??0?10?0????1??000?1000??????????0?10?0?10???1??0??01?00?????1?00?0?0???11?????????00?0100????0?0??11?????00?????????0?00????0?10?0??0?010??00??01?00?00?00?????????????1???????????????1??011?????0???11??10?000??????????1101100??1??????00?0???1???????????00?1??0??10?1?00???0?1?????????0?????????0??0????????01??0???101???10????0001???????????????????00??01?????0???1?101?00????010???0??0?1?0?0?????????00??????1??????0?0?????????????????010?001??????0????????00??00?000????????1???11??1????1100000???0?0????0???11??1?????1?1???0???0000????????1??1??01???1?0?0????0?????????10??0???00????1??0?0??1???0???0???000???0?????1?11?000?1001000????1?????00??????????10?1001??0101?00?0?0??0???000??111010000????????????0??0???0?0?????????????100?01100????00?00????????0????????????????????????????????????100?0???????0?000?????1????????00???????0???????0?????????0?0?????????????0??000000??????0??????0??????0?????0?????0???0?01??????0????????????001??11??1?00?11????????0000?0???000????????????00?????0??0??1????000101?0?1?1??????????????????0?00?????1??00????0???000?01???00000????0???00?????????????0???000?????1?????00?????????????????????????????0???0?0?000???????????????11100000????0?????00??????????0??000?????????????????0?????????1???0??0?0???0??????????0????????0????1??0????????????1100???????????????0?0???00??????0?????0?0???????00????00????????????????????0001???????????????????0??????000?1????10??1
Gualicho (Apesteguía et al., 2016)
626 – ? 229 - 0 368 - ? 1903 - 0
???????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????00???????????0??0??1110010?0?0??00?1???00100?010011000?0?001??0110??100110?0011000001?1??01?????????00?????????????????0?0????????????0110???????????????????????????????????????111??000????????????011111??110?100?11010?110??0??00?????1????????00????00????0?????????????1?0?01?0?????????1????????0?0?0???????0???????0??0?????0??1????0????????0??????????0???0??0??00?0?????????0??0?0????0????00?0????0???0?1????00???00?????????00??1??001????000???????0???10?0???????????????0?0???????????????????1000??????????????0????0???????????0???10?????????0??0???0??0?????0???1??0??????10?????????????????0????0??0?????????????1????????0????????????0??????????????????????????1?010?0???0??0????????0???0?0?0????0?????????????1??0??????01??1?????0?????????????0?1?1????????????????0??0?000???0?0?????0?????000??1???0?0?????0????0???????????????1??00?1?0?????????????????????1??0?0???0000???????10?000?????????????0??????0?????????????0?????????00?0????1?0??1?????????????????????0?000?0???????????00??????00????0???????????????1??0??????0??????????????????????00????1??0????????????????????????0????????????????????????????????????1????????????????????????0???00??????00????????0?????????????10?0?0??0?????????????1??????1???????????????00????????????????????????1??????0?11??????0?0??001????00????????0??0????0??0???????????????????0?????????????????????????????????????????0011?01?????00?????????0?????????????0?????1??????0????????????????????????????????????0???00??0???1????0000????????????????????0?0???????0??????????0?????????????????????????01???0????0???0???1???????????????????01??0??????0??????0?1?????????????0??0?????0?00?1?????????0???????0??????????????????????????????1??????????????????????????????0?????1???
Aerosteon (Rolando et al., 2021)
1605 – 1 942 – 0
????????????????????????????????????????????????????1???????????????????????????????1??000001???1000010??????????????????????????????????????????????????????????????????????????????????????????1100????????10110100111011000?1010110?100001001??11?000101?1001010100000?1100??????????????????????????????????????????????????????????????????????0?1???1??00??????110?0001??????1?0?01?1010001111001101000001001101011010000100????????????????????????????0?000101100000111010011?111????0?????????????????????????????????????????00???1???01?????????0??????????????000?0?1??????????????001?00???1?????00???0??????00?0????0?0??????????0?1?1?1???????0?????0???0????0??00??0??1????1?1??????????0??00????1??????????????00?1?????0?????????00?0???????????111?????01??????????10?11?????1?0????0?0?0?0???????0??????0?????0?????100?1?1?????????????????0?1??1????????11??1?1?0??0?0100???00?1????0??100?????????????000?0??????00??0?10?????0????1?0?0?00???????????00?????00?????10????????0???00?????00??0?0???0?0?????????1????????1????????0????001??0?0?????0????0?0?????1??????10???11??1???????001??????0?0??1???????1?0?00???00????????10??0???0?????00?00??1????0??01?????0?????????11?0??0??00??111???1??????10???0???0??0?000??1?01???01???0??????0????10???0010???????????????0??????????????????000???0?0???000??00?0??000??????????????00???????0??????????????111?????????0?111?????0?????????????????0???????000???????0????0??1?????000??0?000???????0?????????????01111?0?????????1?0?????????0?????0110?00??10???0?0?????????????0???????0?????0????????000????00?????0???000??110?1????????????????????01?1?0?00011?????011??????1??1?01??0?0010??????????????00001?0?000?1???00???????0??????0???1????????????????????0????00?????????0?????11000000????0?0????1????0????????10??000101001101??????0?????0?1??00????0???0??10??????1??????110???0?1???????????????????????????????011?????00????0???????0?????????0???000????????????????????????01?????00?00010??????????1?000?1???????0?
Megaraptor (Calvo et al., 2004; Porfiri et al., 2014)
1605 – 1
?????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????1???????????10110100110001??0?0????1????????????????????1??10010??100010011000?1010?0????11000000?1?01101010011000110001?00101111110100111001????????????????0????001?0???0???????1?0???????????????????????????????101???1000???????????????????????????????????????????????????????????00?????1????1??1100?10?????????????????????0??0?1???????0???????1?0????????0???????100??????00???????????????0???0???????01?????????00?10????????0?????1???????1???0100????100??????????0?0??101?1000???00???????0?0?0???????????????0?????????????????????1000?????????????????010???????????1?1???????????????0???00?0?????0???1??0??????00???????????????????0?100??????1????????11?1?????????????????????????????????????????????100?000???????????????????0?00?0?0?1????????????01??0????????????????????????????0???1???????00????????????0??011?0110?????0?????11???1????0???0??0????0??????????????1????????01??????????0??1??00???1?0?00?????0?11010??????0???????1?0?0????????0???????????????????????0??00?????1???1???????1???????????????????0??????????0????????0??1010??????????????0?????????0????????????????1??????0???????00?00?????0?0????????????0???????????????????????1????????????????????????????0????????????00??????00????????0????????????00??0?0?00??????00???????????????0?????0???????????????????????????1?????0?????1000??????0??????0??????????????0??0????0?10?????0??????????????1???????????????????01?1?0????????????1?1??1?1?1???01?????????0????????????????????????????0???????????????????1????????????????0??0?????????????0000?????????????0???????10?0?00??0??????????????????????????????????????????????0????????11????1?????????0?????????????0????????????????????????100?1??????0????????????????????0????????????????????????????0???????????????????????????0???????1?0??
Murusraptor (Coria & Currie, 2016)
1576 – 1 942 – 1 1605 – 1 1944 - 0
?0??????????????????????????????????????????????????10011?001??0???0???11010?01??11????00000????110000???????1000100011?1?00010010?001?1?????????????????0????000?0?0?????111??1?????1??00001001???????????????????????????????0?????????00??00???100?0001??????????????????????????????????????????????????????????????????????????????????????????????????????????011??????????????0??1?10101011010010010100?10??101?????10?????10??0?0001??????????????????????1101100??01????1??????????????????????????????????????????????????0?0?1???1???????0?1??0?0???1?????????1??????1????0??0?00?1???1??0???1?????001????????000?0???1??0?01?????????1?1??011???0?????000??????????0???0?0?????1????????????1?1?1????0?0???0???????????????????1??01???????????????01??1?1????0???????000?10?1????0?1?0???0?0???????????0???????????001?0??0???010???0?????????????0??????????00?1??????0?0?1???101??????0?0??0?1?100??0??0??0??????????????0???0???0??????0????0???00???????????10?????0?????111????????0?10000?0???1?????0000?????0?0???0?0????????????0?????????1????0?1???0???0?????????????1?1?????1???????????0?1????00??????0?????10?0???????????????11????????????????0???0???????1?????011??1?0????????0??00????1?1??????0?10???0001??????0?????1????????0?????1?0?????????001??1????0????????0?????????????0???????0??0?????00???????????????????????0???????????0?0???11?00?000????????11?1??1??100??????00??0??0?0?0?????????????????1??00??????0??????0??0??????????0??????????????????1??????1??00???0???????????????101????1????1????????1?0110?1?????????????????????????0?????0???1???01??????????0??????010????1???0?????1?????0???????0?1????????????1??0?????????????????0?00??1?????0?1?????????????????????????0??????????????????0?????0?????????????0????????????????????0?????????????????00????0111???????10???????????????????0?0????????????????11??????1?????????????0????00???????????00??????00???????0???????????????????????????????????????????????0??????????????????1???0????1????1?1??0
Maip (Rolando et al., 2022)
221 – 0 224 – 1 232 – ? 748 – 1 1576 – 1 1235 – 1 1185 – 1 942 – 1
??????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????10?10011100?????????1??0?00??1??011???0000000?0??0??0?01?????????100010?1000??????????????????????????????????????????????????????????????????????????????????0???01???????0000????0???????????????????????????????0?????10???????????????????????????????????????????????????????????????????????????????????????????????????????????0??????????????????????????????????????????????????????????????????????????0?????????????????????????????1?????????????????????????????????0???????0?0???????????????????1?????????1?????????1????0??????????????????????1???1????01??????????10????????????????????????????????????0????0??????????0???????????????????????????????????????????????00??????0???????????????????????????00??????????????????0????????00??0???????????10??????????????????????????????????0?00??????????????????????????0???????1????????????????00?????0?0??????????????????????????????0??????????00??0?????1??????????????????????????????0?1??????????????????????????????1?????????????01???????????1?????????1???????????????????????????00???1????011????????????????0????????????????10????0?????????1??0????????????????????????????????????????????????10????????0001??????????????????????????????????????????0??????????????????0?0?0?????????0???????????0???????????????????????????????????????0?????????0????????????0?????????00???0????????00??????????????????0??????????????????????????0???1?1?????????????1????????????1??0???????????????????????????0???????0????????????????????????????????????????????????????????0????????????????????????1?????????????1?????????????????????????????1??????????????????????01?????????????00????????????????????????????????11????0??????01??????0?????????0?????????????????????????????10??0????????????????0????????????????????
Deltadromeus (Sereno et al, 1996)
????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????????10011?1100000?1000100000010001100?0??0??????????????????????????????????????????????????????????????010???????0??0110??????????????????????????????????????????????????0????1?1001111100111000000101101????00010?1??0101????1?00??0?0?0000001100??1?1????0???110????????0?????????1???????????0?????1??????????0??1??1??????????1??0???????????0??0??00?????0??????0???1????????000??11?10?????1??1??0????????????1??0????1?0?1?????0?0?????1???1?000???10??????????0??1??????0???????????011?????0?????1??0???????????????????000?0???????0?????????????????00???00???1?1?0?????????????????????????????????????????1???1001?????????0?0????????????????????????????0??????????0?0?????001?????000??10???????????????????????????1?????1?????????????1?1?1?????010?????1??0??????0????????1?????0?????0?1100000?????0????0???????????????11??0?1????????????????????0??0??0?0?????00???????10?0???1?0?????????0??1???0???????0?????0???????0??0?0????1????1??1??????????????????0??00?????01????1?0??0????0????????????????????????0?0????1?0?????????????????????????????00?00???????????0??????????????????????????????????0??????????????????????????0????????0??????????????????????????????????????0?0??????????1????11????00??????????0?????00???0???00???????????????1???1??????????????0??00?????00????????????????0??0?0??0????????????????1??????????????????????????????????????????1?01???????????????100??????????????????1??????0?????????????????????????????0??????????????????????????0?????????????????0?????00??????????????000??????????????1?0?????????1???0??0?0???1???1???????????????????00?0??????????????1?0?????0???0?000?0?000??0?000??0???????0??????????0?????????????????????????011???????????????????0???????????????11111
In addition, characters 891, 1453, and 1285 were changed for Phuwiangvenator, to 0, 0, and ? respectively (Samathi et al., 2019), and for Torvosaurus tanneri, characters 347, 372, and 1843 were scored as ? (Rolando et al., 2020).
A modified matrix from Cau and Paterna (2025) was used to determine the phylogenetic position of Bahariasaurus and its presumed phylogenetic affinities with Deltadromeus. The analysis focused on non-maniraptoriform theropods and included a broad representation of Abelisauroidea. The analysis produced a majority-rule consensus (>50%) from the 2851 most parsimonious trees. Tree lenght of 4587 steps.
As shown, Deltadromeus, together with Gualicho and Elaphrosaurus, forms a clade within Abelisauroidea in all resulting trees, whereas Bahariasaurus occupies varying positions within Megaraptora. It takes 5 additional steps to force Bahariasaurus into a clade with Deltadromeus, Gualicho, and Elaphrosaurus. While this is not a high number, it primarily reflects the incompleteness of Bahariasaurus and other key taxa. To illustrate what 5 steps mean in the context of the matrix, it takes only 3 additional steps to place Bahariasaurus as sister to Eotyrannus, also 3 steps for Torvosaurus, 4 for Yutyrannus, 7 for Neovenator, 3 for Coelurus, 8 for Berthasaura, and likewise 8 for the Angeac taxon.
Due to the high instability of Bahariasaurus, this analysis on its own does not prove anything conclusively, but it clearly illustrates that Bahariasaurus is not phylogenetically closest to Abelisauroidea, specifically Deltadromeus, but rather to coelurosaurs, specifically representatives of Megaraptora.
To further expand the comparison, the skeletal material of Bahariasaurus will be evaluated element by element in comparison with Megaraptora and Abelisauroidea.
Overview of Bahariasaurus material with focus on Megaraptora and Abelisauroidea clades
Megaraptora, whose record in Africa is very limited, however, their presence would be expected due to their wide distribution across the rest of Gondwana. Megaraptorans in Africa are represented by some footprints and two incomplete caudal vertebrae. Both records are most consistent with megaraptorans, although a spinosaurid affinity cannot be ruled out. Regardless of the identification of these fossils, megaraptorans are present in Gondwana, geographically and temporally close to Bahariasaurus.
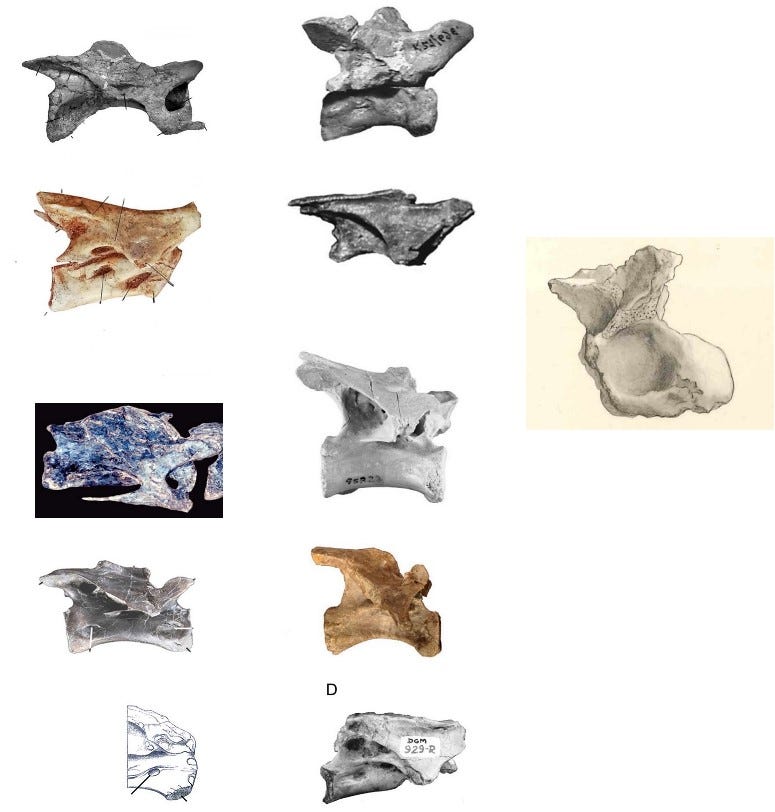
Most importantly, several of these forms exhibit features that are nearly unique to Bahariasaurus + Megaraptora. This concerns the sacral and caudal region. The sacral vertebrae of Bahariasaurus are elongated (twice as long as high), wide (50% wider than high), and possess pleurocoels. This combination of features is present only in some oviraptorosaurs and an unnamed megaraptoran from the Albian of Brazil – SMNS 58023 (Frey & Martill, 1995; Rolando et al., 2018). Aoniraptor libertatem is also very similar, possessing the same degree of centrum elongation and pleurocoels, although the centrum is not as expanded. Pneumatization of the sacral centra is absent in all known members of Abelisauroidea.
Another aspect linking Bahariasaurus to megaraptorans is found in the caudal vertebrae. The first point is the presence of pneumatized centra, a condition that among theropods is seen only in Oviraptorosauria, Therizinosauria, Megaraptora, Berthasaura and Carcharodontosauridae. (Rolando et al, 2020). Clearly, Bahariasaurus does not belong to Carcharodontosauridae, Oviraptorosauria, or Therizinosauria, which is rather evident when comparing the totality of available fossil material. Megaraptora correspond to Bahariasaurus not only in the presence of pleurocoels, but - just as in the case of the sacral vertebrae - also in the elongation of the centrum. The anterior caudal vertebra of Bahariasaurus (IPHG 1912 VIII 62) in general appearance is very similar to the first caudal vertebra of Aoniraptor, as shown in the figure below. The main differences include a more developed anterior chevron facet and the presence of a ventral groove in Bahariasaurus.
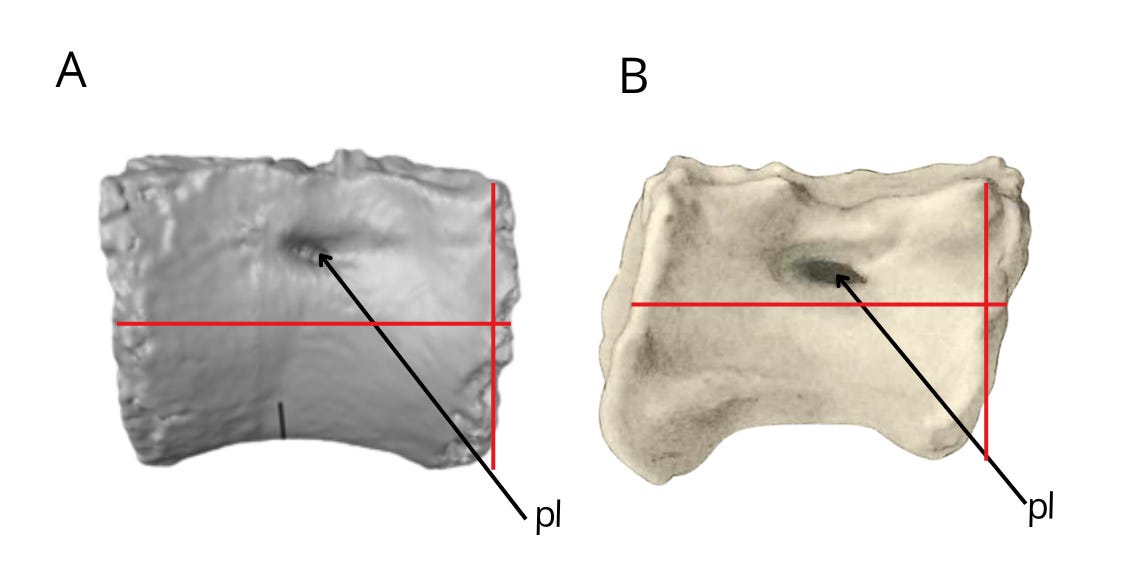
Elongation of anterior caudal centra and the presence of pneumatic openings are also seen in the vertebra UFRJ DG 558-R from the Açu Formation in Brazil (Pereira et al., 2020). Pneumatization of caudal centra in Abelisauroidea is present only in Berthasaura (de Sousa et al, 2021), but this taxon clearly differs from Bahariasaurus in lacking such pneumatization in the dorsal and sacral vertebrae, as well as in several other, important features of the pectoral girdle, pelvic girdle, and cervical vertebrae.
The highly developed pneumatization of Bahariasaurus (dorsal, sacral, and caudal vertebrae) is extremely unexpected for an abelisauroid.
The similarities in the caudal vertebrae between Bahariasaurus and megaraptorans do not end there. Middle caudal vertebrae of the Egyptian taxon are also known, which - as has been demonstrated - differ significantly from those of Deltadromeus but are strikingly consistent with megaraptoran morphology. This can be seen by comparing Bahariasaurus with the megaraptorans Aoniraptor, the unnamed megaraptorid NMV P257414 (Kotevski et al., 2025), and the probable megaraptoran NMV P252704 (Poropat et al., 2019). The middle caudals of all these taxa share short, dorsally projected prezygapophyses, neural spines in the form of a low ridge extending towards a slightly dorsally projected postzygapophysis, well-defined transverse processes located at the boundary between the centrum and neural arch, and likely the presence of a lateral ridge.
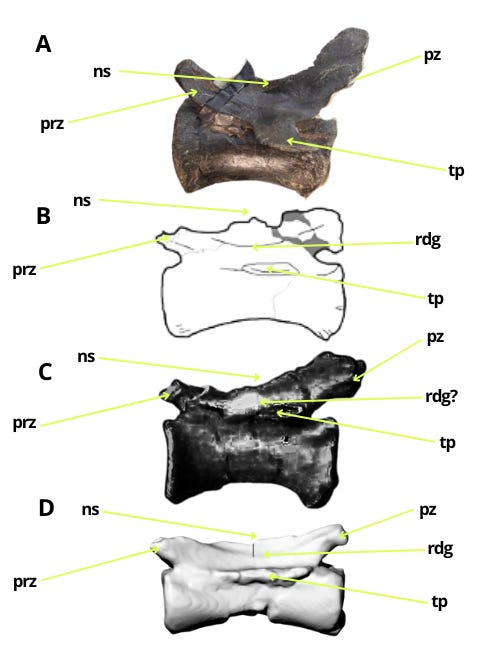
All these vertebrae form a more or less coherent morphotype, which significantly differs from the morphology of the caudal vertebrae of Deltadromeus + Gualicho + Elaphrosaurus. All the remarks regarding differences between Bahariasaurus and Deltadromeus can be applied to the entirety of these morphotypes.
When discussing the caudal vertebrae of Bahariasaurus, it is worth noting that the illustrated vertebra of specimen IPHG 1912 VIII 60 shows structures resembling fossae, which are delineated by the described centrodiapophyseal and prezygodiapophyseal laminae. If this observation is correct and the taxon indeed possessed fossae in the caudal neural arches, it would be another significant indicator, as aside from Megaraptora, only a few taxa exhibit such features, and their complete absence is characteristic of Abelisauroidea.
In summary, the morphology of the sacral and caudal vertebrae of Bahariasaurus strongly resembles the condition seen in representatives of Megaraptora and at the same time significantly diverges from what is observed in Abelisauroidea.
Furthermore, the overall skeletal anatomy of Bahariasaurus seems to correspond more closely to that of megaraptorans than to abelisauroids, although in many cases comparisons are hindered by the incompleteness of basal members of the former group.
The cervical vertebra of Bahariasaurus is strongly opisthocoelous, relatively short anteroposteriorly, and bears a single pneumatic fossa laterally. All these features stand in stark contrast to the morphology of cervical vertebrae in basal abelisauroids, as shown in the figure below. Abelisauroids had platycelous centra, with remarkable elongation and with is characteristic, two pneumatic openings (on anteriorly, second posteriorly) in each centrum. Also the neural arch is very different in Bahariasaurus in being anteroposteriorly shorter, less dorsally flattened and possessing large postspinal fossa.
All these features seen in Bahariasaurus are common and diagnostic for Tetanurae and completely absent in Abelisauroidea.
Cau argues that the scapulocoracoid of Bahariasaurus is “clearly abelisauroid” based on: the dorsoventrally expanded acromion, the enlarged elliptical supraglenoid fossa, the low position of the coracoid foramen, the lack of the biceps tuber, the moderately elongate posteroventral process, the inclination of the acromion relative to the shaft, the lipped margin of the acromion, and the distal narrowing of the shaft. I'm not sure whether in this context dorsoventrally expanded acromion refers to the direction from the junction with the coracoid dorsally toward the scapular shaft (which is how I would interpret it), or anteroposteriorly when looking at the scapulocoracoid in its natural anatomical position within the body. Either way, it doesn't make much sense. If it's referring to anteroposterior expansion, there's no real discrepancy compared to most theropods. However, if it's supposed to be dorsoventral, then it's laughable as in reality, this is one of the clear features distinguishing it from Deltadromeus. Moreover, this trait contradicts the basal abelisauroids in question - Deltadromeus, Elaphrosaurus, Limusaurus, Berthasaura, and likely Vespersaurus. All of them exhibit a dorsoventrally low acromion. In fact, even if we reconstruct the acromion of Bahariasaurus differently than in Fig. 2, with a marked anterior lowering similar to the condition seen in Aerosteon, it would still remain distinctly higher - at least compared to Deltadromeus. In any scenario, Bahariasaurus possessed a high acromion, which is commonly visible among large-armed theropods such as Falcarius, Therizinosaurus, or Deinocheirus, and is also similar to Aerosteon. The enlarged elliptical supraglenoid fossa is characteristic of abelisauroids, occurring in Masiakasaurus, Limusaurus, Deltadromeus, Elaphrosaurus, but probably also Deinocheirus. Its presence in Bahariasaurus can only be assessed based on illustrations. Indeed, something resembling a fossa can be seen; however, this interpretation is not unequivocal. In fact, Stromer explicitly describes a distinct thickening of the scapula in its posterior part, starting just above the junction with the coracoid. This thickening may correspond to a supraglenoid flange and, together with more central convex tubercles and grooves (probably of muscular origin), create the illusion of a “fossa.” In this case, it is not a “fossa” that lies below the level of the coracoid, acromion, or scapular blade, but rather various thickenings and protrusions rising above that level, contrasting with the flat surface of the scapula. The supraglenoid fossa in Deltadromeus and Elaphrosaurus is extensive, extending onto the coracoid fused with the scapula. The coracoid of Bahariasaurus is not available in lateral view, so even if the interpretation as a fossa is accepted, its analogy to that in Deltadromeus and Elaphrosaurus remains unclear. The low position of the coracoid foramen appears simply to be untrue for Bahariasaurus, since the foramen lies right at the boundary with the scapula and its position is consistent with that observed in many theropods. The absence of a biceps tuber is also unclear for Bahariasaurus as there is no lateral view of the bone. However, Stromer in his description mentions a thickening present posteroventrally relative to the scapular articulation - this structure could correspond to the biceps tuber. It is worth noting that both the absence and presence of this structure are consistent with megaraptorans, where basal forms (Vayuraptor, Fukuiraptor) lack it, and derived ones (Megaraptor, Maip) possess it. The moderately elongate posteroventral process is a common feature, consistent with many clades, and does not contribute much to identification. The inclination of the acromion relative to the shaft is indeed consistent with abelisauroids, but also with basal coelurosaurs including megaraptorans and many other clades, so again it is not unique. The lipped margin of the acromion is unclear to me, as the edge of the acromion is not preserved. The distal narrowing of the shaft is also unclear. Firstly, the bone is damaged, which affects its appearance in the illustration; secondly, Stromer notes that the shaft expands dorsally rather than narrows. It should also be noted that the bone is incomplete and a large dorsal portion is missing. Noteworthy, Stromer refers partial scapulocoracoid IPHG 1912 VIII 77 to the same kind as IPHG 1912 VIII 60 (Bahariasaurus here), unfortunately there is no illustration and stated characters aren’t convincing. However, if this element is taken into account, Stromer notes that the dorsal portion of the scapula is complete and doesn’t show a distinct expansion. At the same time, the shaft does not taper but remains approximately the same width along its entire length.
In other words, there is no convincing evidence supporting an abelisauroid affinities for the scapulocoracoid of Bahariasaurus. In reality the pectoral girdle of Bahariasaurus resembles that of megaraptorans: the coracoid has proportions similar to those of Megaraptor and NMV P186327 (Benson et al., 2012) and the same time it strongly differs from anteroposteriorly expanded coracoids of abelisauroids; the glenoid is enormous, which, combined with a large acromion surface (attachment site for muscles controlling the humerus) and large muscle scars, suggests powerful forelimbs - again consistent with megaraptorans. At the same time, abelisauroids are fundamentally different, as they are known for their reduced forelimbs.
Comparison of the dorsal vertebrae is more difficult because in megaraptorans these elements are mostly preserved only in derived members, which are not perfect points of reference. The dorsals of Bahariasaurus are morphologically intermediate between the basal Asian megaraptorans and the derived Gondwanian forms. The proportions of the Bahariasaurus centra are nearly identical to those of Fukuiraptor (Azuma&Currie, 2000) and especially Phuwiangvenator (Samathi et al, 2019), while the presence of pleurocoels strongly resembles the more derived forms. The structure of the neural arch is not known in basal megaraptorans, making comparison impossible but in general appearance its morphology match both - basal abelisauroids and basal coelurosaurs.
The pelvic girdle is the element that, at first glance, most clearly distinguishes Bahariasaurus from known megaraptorans; however, again, comparable elements come from derived representatives, and data for this region in basal forms are lacking. The pelvic girdle of Bahariasaurus consists of the pubis, ischium, and a partial ilium. Unfortunately, the latter element has not been illustrated, and its considerable damage and incompleteness have been noted. However, some information has been provided. The dorsal edge of the ilium is very straight along the entire preserved length; in the posterior part of the bone, the dorsal and ventral ridges converge at a sharp angle, forming a “spike,” similar to that seen in Megalosaurus. Additionally, a very short ischiatic peduncle with an almost circular termination was recorded. These fragmentary details preclude deeper comparison, but the posterior “spike” is unusual for both abelisauroids and megaraptorans. The next element is the pubis. In lateral view, the shaft is straight, a condition widespread among theropods; thus, it is present in Berthasaura, Elaphrosaurus, megaraptoran NMV P186046 (Benson et al., 2012), Aerosteon, but not in Masiakasaurus, where the shaft is anteriorly convex. Proximally, the pubis is distinctly longer anteroposteriorly than dorsoventrally tall, forming a sickle-shaped structure. There are no laminae ventral to the ischial contact, thus lacking an obturator foramen and likely any notch. This is unusual for abelisauroids, which exhibit an obturator perforation (e.g., Berthasaura, Vespersaurus, Limusaurus, Masiakasaurus). The closest analog among abelisauroids might be Elaphrosaurus, though this is unclear because the bone is incomplete and some kind of perforation might have been present. In this respect (sickle-shaped proximally pubis with no obturator perforation), ornithomimosaurs are anatomically the closest. Megaraptorans have a more compact proximal morphology but also lack a distinct obturator foramen. A proximally positioned interpubic fenestra is present, which appears widespread among Tetanurae, but within Abelisauroidea is likely only found in Masiakasaurus. The fenestra is small and oval, dorsoventrally higher than wide, and its placement and size resemble NMV P186046. It should be noted that the pubis of specimen X47 is distally flared, while the pubis of specimen VIII81, despite being half the size, exhibits full fusion. Stromer suggested that the latter is most likely a juvenile Bahariasaurus, attributing the differences to ontogeny. In general appearance, both specimens are very similar, but this difference could be ontogenetically problematic and calls into question the referral of specimen VIII81 to Bahariasaurus. The morphology of the distal pubic boot in Bahariasaurus depends on interpretation of the aforementioned pubis. In the larger pubes, the pubic boot is incomplete, so little can be said beyond that it was most probably small. Specimen VIII81 may present a fully preserved pubic boot if its referral is accepted. In that case, it appears slightly expanded posteriorly and somewhat more anteroventrally. Such morphology is not consistent with abelisauroids, where the pubic boot is small but not projected anteroventrally to this extent. Known megaraptorans differ by possessing a large, well-developed anteroposteriorly elongated pubic boot; however, it should be noted that this element is unknown in basal megaraptorans, so the evolutionary timing of this trait within the clade is uncertain. The next element is the ischium, which, thanks to the identification of specimen VIII82 as Bahariasaurus, can be considered as almost complete in this taxon. The entire element is slender and vertically straight, with a relatively elongated iliac peduncle, a moderately concave acetabular margin, and a triangular obturator process lacking a distinct notch beneath it. These features generally align with coelurosaurs, while abelisauroids usually differ noticeably. Abelisauroids often have a dorsoventrally tall pubic peduncle and an obturator foramen (e.g., Limusaurus, Berthasaura), and the obturator process forms an extensive flange rather than a triangular shape as in Bahariasaurus. Elaphrosaurus is very different, with a more robust element, lacking a triangular obturator process but having an extensive flange, a larger pubic peduncle surface oriented more dorsally, a deeper acetabular margin, and a medial crest on the posterior margin of the shaft. The most unusual feature is the distal morphology of the Bahariasaurus ischium, which resembles basal theropods more than coelurosaurs. It has an anteroposteriorly expanded ischial boot, seen in Masiakasaurus, Deltadromeus, Elaphrosaurus, Megalosaurus, Concavenator, Neovenator, and Alpkarakush, with the greatest similarity in boot inclination to the first three. Bahariasaurus differs from Deltadromeus, Masiakasaurus, Alpkarakush and Elaphrosaurus, but resembles Neovenator (Brusatte et al, 2008) in having posteriorly unfused ischial boot, which may be ontogenetic. Overall, the ischium - specifically its distal end - likely represents the strongest argument not only for the proposed synonymy of Bahariasaurus and Deltadromeus, but also for potential abelisauroid phylogenetic affinities of the former. However, considering the scarcity of this morphologically matching element and the limited number of shared features, when contrasted with the rest of the Bahariasaurus skeletal material, which clearly deviates from abelisauroid standards, this interpretation does not appear convincing.
Overall,
The skeletal anatomy of Bahariasaurus overall seem to aligns better with megaraptorans than with abelisauroids, although incomplete fossils of basal megaraptorans make some comparisons difficult. Its cervical vertebra is short, opisthocoelous, and bear a single pneumatic fossa, contrasting sharply with the platycoelous, elongated, and doubly pneumatized cervicals of abelisauroids. The scapulocoracoid has been argued to show abelisauroid features, but many such claims are either incorrect, unclear due to preservation, or equally consistent with megaraptorans. For instance, large glenoid and muscle scars are evidence of strong forelimb musculature that is more typical in megaraptorans. The dorsal vertebrae of Bahariasaurus show centra proportions similar to basal megaraptorans like Fukuiraptor and Phuwiangvenator, with pleurocoels resembling more derived forms. Pelvic elements are more difficult to interpret due to limited comparable material. The pubis lacks an obturator foramen, which is rather unusual for abelisauroids and has a morphology closer to ornithomimosaurs or megaraptorans. The ischium, while sharing a distally expanded boot with several taxa (including mainly abelisauroids and some others), shows overall features more typical of coelurosaurs. Though the ischium’s distal morphology has been cited as evidence for synonymy with Deltadromeus or abelisauroid affinities, the rest of the skeletal anatomy contradicts this, supporting a closer relationship to megaraptorans.
Short comments on some other Stromer’s material
Stromer described a large (122 cm) femur IPHG 1912 VIII 69 and a large (104 cm) fibula IPHG 1912 VIII 70 and, based on differences relative to other gigantic theropods from Bahariya, tentatively assigned them to Bahariasaurus. Obviously, this material does not belong to the Bahariasaurus type material, and its assignment is impossible due to the lack of femur or fibula in the type or referred specimens. Over the subsequent years, these two enormous bones have been the subject of debate regarding their identification, with Sereno et al. (1996) and Ibrahim et al. (2020) both considering the elements indistinguishable from Deltadromeus and suggesting they should be assigned to that taxon. It is worth noting that both elements were not found in association, so in theory, they could belong to two different taxa.
Interpretation of the femur and its association with Deltadromeus is complicated mainly due to the poor illustration and description of the latter. Bahariasaurus unquestionably shares with Deltadromeus a prominent medial condyle, also present in Ekrixinatosaurus and Teratophoneus, which was proposed as an autapomorphy of the Moroccan taxon. Another proposed autapomorphy is the presence of an additional trochanter, the anatomical identification of which has been questioned; some authors suggested it is a common lateral ridge among ornithomimosaurs. Ibrahim, Sereno, and others (2020) defend the original interpretation and autapomorphic nature of the described trochanter. They describe the structure as present on the latero-posterior edge, below the fourth trochanter, extending exclusively posteriorly. However, they do not provide any figures of this element. The femur of Deltadromeus was illustrated in posterior view by Evans et al. (2015), where indeed, below the fourth trochanter, centrally and slightly laterally, a certain structure can be seen that may correspond to the described trochanter. If so, identifying this feature in the Bahariya femur is difficult since we do not have its posterior view. From the lateral view, no clear structure is visible that could correspond to the trochanter described in Deltadromeus. Comparison is further complicated by the lack of lateral view illustrations of this femoral part in Deltadromeus. Generally, the nature of the "accessory trochanter" in Deltadromeus and its presence in the Bahariya femur is a problematic issue that requires better description and illustration of the Deltadromeus material.
Based on available illustrations, significant differences can be observed, as shown in the figure below. The femoral head of Bahariasaurus is strongly anteriorly projected, which cannot be said of Deltadromeus, where the femoral head aligns directly with the greater trochanter. In Bahariasaurus, the fourth trochanter is relatively small and does not reach the height of the lesser trochanter. In Deltadromeus, the fourth trochanter is extensive, reaches the height of the lesser trochanter and is generally more proximally placed. These differences are unlikely to be explained ontogenetically. Overall, taxonomic identification of the femur based on currently known information is impossible. At present, this material cannot be confidently referred either to Deltadromeus or to Bahariasaurus. If the structure visible in Bahariasaurus turned out to be analogous to the autapomorphic additional trochanter of Deltadromeus, then, combined with another rare feature such as the expanded medial condyle, the weight of evidence would probably favor assigning this bone to Deltadromeus agilis.
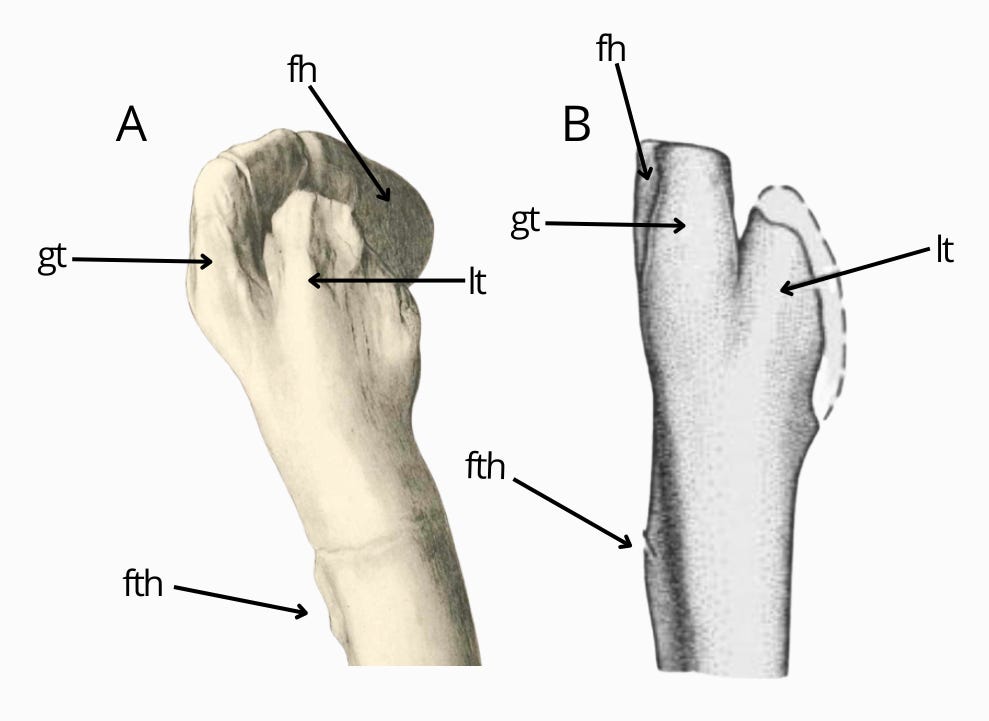
The gigantic fibula has also been assigned to Deltadromeus by Sereno et al. (1996) and Ibrahim et al. (2020). However, comparison here is complicated by the fact that the illustrated fibula of Deltadromeus is only a proximal fragment. The fibula from Bahariya is indeed similar to Deltadromeus; however, the latter has a better-projected proximodistal end and a less extensive fibular fossa, especially proximally. A large fibular fossa and a narrowing below the attachment of the m. iliofibularis are in no way unique to these two taxa. These are common features among coelurosaurs and convergent with gracile-limbed abelisauroids. Therefore, the fibula from Bahariya cannot be identified as Deltadromeus agilis, and its morphology corresponds to both abelisauroids and coelurosaurs. Should be noted that fibular fossa in Bahariya's fibula is very large, exceeding the condition visible in most theropods including abelisauroids and coelurosaurs.
Another interesting find described by Stromer is IPHG 1912 VIII 63, which includes seven caudal vertebrae and a long bone fragment, probably a metatarsal. Four of these vertebrae are from the anterior-middle portion of the tail, while three are from the posterior caudal region. The anterior vertebrae are amphicoelous and measure between 11 and 12.3 cm in length, being roughly as tall as they are wide. Laterally, they lack pleurocoels; however, some depression is present, so it is not excluded that the more proximal vertebrae had some pneumatic openings. This is especially likely because the illustrated vertebra has a transverse process at the boundary between the centrum and neural arch, which is characteristic of more middle caudal vertebrae. Ventrally, a groove is present, and little can be said about the neural arches; the neural spine appears relatively small and spike-like. The remaining vertebrae come from the distal part of the tail, based on their smaller size and complete lack of transverse processes. It is worth noting, as Stromer already observed, that the distal vertebrae differ from most known theropods by lacking elongation of the centrum characteristic of the distal tail segments. These vertebrae are only slightly longer than tall and also do not show dorsoventral flattening, as the centrum width is close to its height. Interestingly, the illustrated distal vertebra, like the more anterior one, has a lateral depression on the centrum. All of these features distinguish VIII 63 from described theropod taxa from Bahariya. Spinosaurus differs in the proportions of the centra, lacks lateral depression and ventral groove; Tameryraptor has a lateral foramen but no ventral groove; and Bahariasaurus differs in centrum proportions, presence of a lateral foramen, centrodiapophyseal lamina, and the fact that its caudal centra become increasingly elongated distally. This indicates the presence of yet another middle- to large-sized theropod in the formation. Searching for similar material, the theropod described by Stromer resembles Vb-607, Vb-717, and Vb-870 - three caudal vertebrae described by Rauhut (1995) from the Wadi Milk Formation, which is located in Sudan and dated to the Cenomanian, thus corresponding to the Bahariya Formation. Both forms have similar centrum proportions; the Sudanese vertebrae show a lateral depression, which in the most proximal vertebra is capped by a small pneumatic foramen, and in the most distal is only a shallow depression. A ventral groove is also present. Vb-870, identified as a middle caudal vertebra, like the Egyptian specimen, shows no high degree of elongation. All of this suggests these forms may be closely related and could belong to Carcharodontosauridae, judging by their proportions and the presence of potentially pneumatic lateral depressions. Similar, however different in some ways, vertebrae were also found in the Acu Formation, Brazil. If the above interpretation is true, IPHG 1912 VIII 63 represents the second carcharodontosaurid in the Bahariya Formation after Tameryraptor and another large theropod of this ecosystem.
Stromer also described IPHG 1912 VIII 177 - left and right humeri, IPHG 1912 VIII 182 - left and right humeri, and finally IPHG 1912 VIII 193 - left humerus. All of these bones are very similar to each other, sharing above all the characteristic slenderness of the shaft. Stromer identified the bones as theropod humeri, distinguishing them from crocodylomorph elements. Later, Mortimer (online) suggested that IPHG 1912 VIII 177 is actually an abelisauroid metatarsal (similar to e.g. Elaphrosaurus), but without providing a broader explanation. Recently, Cau and Paterna (2025) once again treated IPHG 1912 VIII 177 as a humerus. I lean towards Stromer’s original interpretation, especially when considering the entire set of bones rather than just IPHG 1912 VIII 177. It seems doubtful that these are metatarsals, as Stromer described a crista deltoidea - a structure typical of humeri. Moreover, in the illustrated VIII 193, it is clear that the bone is expanded proximally only on one side, which does not match the symmetrically widened metatarsus. Another important aspect is the size of these elements. IPHG 1912 VIII 193 is incomplete; the preserved fragment measures 40 cm in length, but by comparing measurements to the complete IPHG 1912 VIII 177 (54.3 cm) and directly overlaying the bones, it can be estimated that, if complete, it would have been ≈ 70 cm long. Such a size seems highly improbable if identified as a metatarsal. A humerus measuring 70 cm would be among the longest known in theropods, surpassed only by Deinocheirus, Therizinosaurus, and slightly by Gigantoraptor. The unusual, even extreme slenderness of these humeri automatically excludes assignment to spinosaurids, carcharodontosaurids, megaraptorans, abelisaurids, or essentially anything outside of basal abelisauroids. These forms are known for slender, reduced humeri, but these are usually relatively short. An exception is Deltadromeus agilis, in which the discovery of complete humeri (Ibrahim et al., 2020) revealed these elements to be extremely slender and elongated. Unfortunately, no measurements are available; however, by measuring the reconstruction published in that paper, the humerus appears to be about 55 cm long, while the entire animal is estimated at 9 meters along the centra. In this way, a 70 cm humerus would imply an animal nearly 11.5 meters long, which is an enormous size for an abelisauroid. Regardless of the estimate for Deltadromeus, IPHG 1912 VIII 193 represents a gigantic abelisauroid with the closest phylogenetic affinity to the Moroccan Deltadromeus. *It should be noted that Stromer described the humeri as weakly muscled and with limited mobility, and at the same time explicitly stated that they could not belong to Bahariasaurus because “they (pectoral girdle elements of Bahariasaurus) do not fit to a reduced forelimb.” This is worth noting, because nowadays Cau sees no issue with assigning at least one of these humeri (VIII 177) to Bahariasaurus.
To summarize, I will begin with something that perhaps could have appeared at the beginning of this post
What exactly is Bahariasaurus ingens?
Without a doubt, it is a mysterious taxon that has long been subject to misunderstanding. Certain assumptions regarding its invalid status or chimeric nature were established years ago, largely without justification - the result of the topic being ignored after the original material was destroyed and have persisted to this day, not only in the media but even in scientific literature. For instance, the claim that Bahariasaurus can only be referred to by the “holotype” specimen IPHG 1922 X 47 is entirely mistaken. In 2008, for example, Andrea Cau wrote on his blog: "Unfortunately, Stromer assigned a number of other fragmentary remains to this genus, although the evidence supporting that referral is very weak, since at least two other giant theropods potentially corresponding to those remains occur in the same formation." This fundamentally flawed assumption seems to have followed the author all the way to 2024… In reality, Stromer was quite careful in his taxonomic decisions. To begin with, he designated a syntype series consisting of IPHG 1922 X 47, IPHG 1922 X 48, and IPHG 1922 X 48 B. All these individuals share overlapping, morphologically consistent material. The first two possess posterior dorsal vertebrae that are of the same size, platycoelous, nearly twice as long as tall, with similarly proportioned articular surfaces, lateral foramina, and the presence of a single posterior centrodiapophyseal lamina - all of these indicating identical morphology. IPHG 1922 X 48 B shares pubes of identical morphology with IPHG 1922 X 47. Thus, the taxon is based on unquestionably associated material. In addition, Stromer referred several other specimens to Bahariasaurus, mostly on the basis of overlapping and morphologically consistent elements. IPHG 1912 VIII 62 represents the largest individual confidently attributable to the same taxon as the syntypes, based on matching posterior dorsal vertebrae and an essentially identical pubis. Another important specimen is IPHG 1912 VIII 60, which preserves parts of the pectoral girdle. Its referral to Bahariasaurus is justified by associated caudal vertebrae exhibiting lateral foramina, ventral grooves, and relatively broad centra. This unique combination of features is not only distinct among theropods from Bahariya but rare in a broader context as well. Since this feature set is present in Bahariasaurus (e.g., IPHG 1912 VIII 62), the referral of this individual to the taxon is well supported. Several other specimens can also likely be referred to Bahariasaurus:
IPHG 1911 XII 23, a proximal ischial fragment virtually identical to X47, but much smaller; IPHG 1912 VIII 74, another proximal ischial piece, again identical to X47; IPHG 1912 VIII 83, consisting of two middle caudal vertebrae that match the morphology of VIII 62, though smaller; IPHG 1912 VIII 82, recently re-identified as an ischium, showing the same morphology as the above. Additionally, an unillustrated scapulocoracoid (IPHG 1912 VIII 77) and less likely a proposed juvenile pubis (IPHG 1912 VIII 81) may belong to Bahariasaurus.
All of this evidence clearly shows that the long-standing notion of Bahariasaurus ingens being a chimeric assemblage is unfounded and highly unlikely.
Another issue concerns the validity of the taxon, which was unjustifiably questioned by Chure, 2000, Ibrahim et al. in 2020, or generally in media. In truth, Bahariasaurus is diagnostic even when restricted to IPHG 1922 X 47 alone, due to the presence of elongate sacral centra bearing pleurocoels and a ventral groove. Taken together, Bahariasaurus specimens exhibit numerous rare features (e.g., caudal pleurocoels; distally flared pubes with a small interpubic fenestra; mediodorsal ischial process; pubis without an obturator foramen; ischium with a triangular obturator process lacking a distinct notch) that, in combination, provide a strong diagnosis. The taxon is unquestionably valid.
In conclusion:
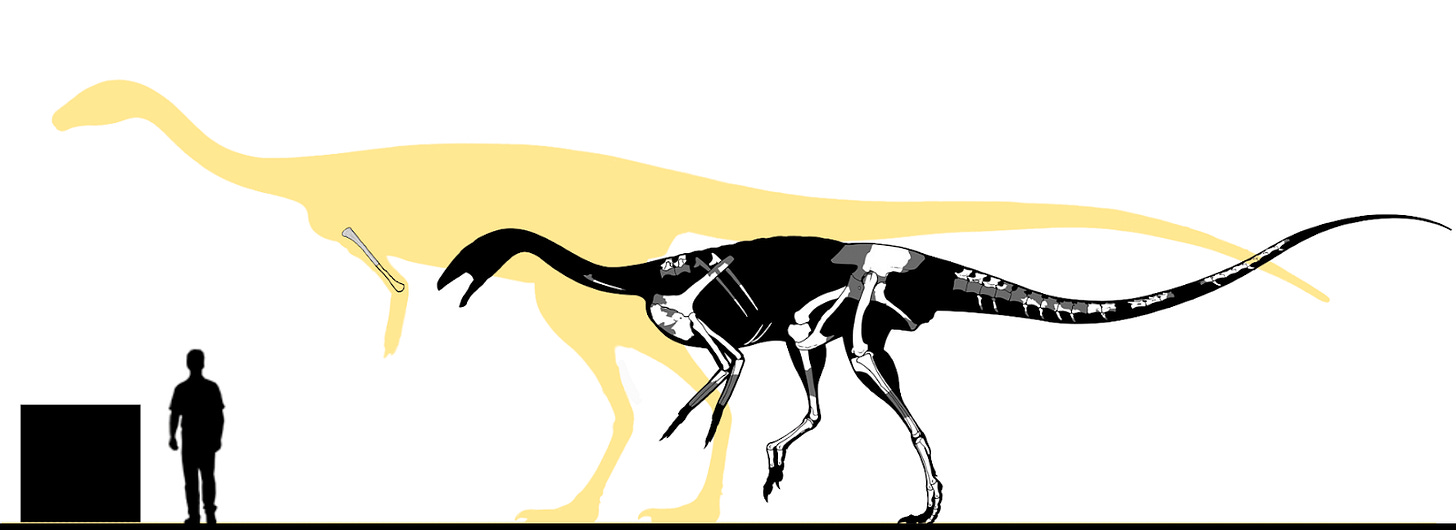
In my view, Bahariasaurus ingens represents a gigantic, valid, non-chimeric coelurosaurian taxon, most likely a member of Megaraptora. Its immense size places it among the largest known theropods, and it was almost certainly the apex predator of the Cenomanian Egyptian fauna - clearly surpassing other theropods present at the time, such as Tameryraptor and Spinosaurus.
Below is a silhouette of Bahariasaurus, modified from Henrique Paes’s Aerosteon model. Known, illustrated elements of the taxon are overlaid, and size estimates are provided for both the type and the largest known individual. We can only hope that future groundbreaking discoveries - whether additional remains of this very species or fossils of closely related forms - will shed new light on this remarkable theropod.
The main purpose of this post was to establish that Bahariasaurus ingens and Deltadromeus agilis are distinct species, by exposing errors in prior interpretations and proposing a fresh framework for understanding the anatomical and phylogenetic identity of Bahariasaurus. Additionally, brief comments were made regarding some of Stromer’s other fossil discoveries.
Edit #1 (20/07/25)
I found paper by Delcourt (2018) ‘Ceratosaur palaeobiology: new insights on evolution and ecology of the southern rulers’ where is a photo of Deltadromeus pectoral girdle and forelimb elements, including humerus. When measured from scale, humerus turn’s out to be 47cm long. Assuming that the whole animal length is 8.5m along the centra, then scaling 70cm humerus (IPHG 1912 VIII 193 commented above in my post) gives about 12.7m in length for Bahariya’s abelisauroid.
I’ve just opened my eyes and noticed that in the photo of the material belonging to the holotype of Deltadromeus agilis – taken and published on Twitter by @AugustoPaleo and used in Cau’s (2025, online) argument – you can clearly see... the pubes of Deltadromeus that were reinterpreted as ischia by Cau (for example), but also earlier by Longrich (online), Mortimer (online), Carrano & Sampson (2008), and Apesteguía et al. (2016). And next to them... ischia? Yes – in the photo, there is an element that matches the shape of the illustration in Ibrahim et al. (2020), and based on its strongly mediolaterally flattened shaft, it can be identified as an ischium of Deltadromeus.
So what about the elements originally interpreted as pubes, which several of the above-mentioned authors later claimed were actually ischia? It’s obvious that the visible pubes cannot “distally complete” the preserved ischial fragment and form a single ischial structure – because, as seen in the photo (e.g., through the nearby fibula), or in Ibrahim et al. 2020a, fig. 118, this element is simply way too large. It exceeds the femur in length – about 150% of femoral length.
The only possible reinterpretation of the pubes as ischia would be to say that only the distal part of the pubes is actually ischial. In fact, as seen in the photo and in the original figure (Sereno et al. 1996, fig. 3F), this part is broken off from the rest of the (fractured) shaft and doesn’t form a physical whole. However, I still find this interpretation doubtful. First, the distal piece “physically fits” the rest of the shaft almost perfectly – this is clearly visible in the photo. Second, even if we assume that only the distal pubic fragment “distally completes” the preserved ischium (plus a few additional centimeters of shaft), the resulting element is still disproportionately large for a theropod as slender and lightly built as Deltadromeus. Also, it’s worth pointing out that the “ischial’’ boot of Deltadromeus is extremely large for an ischium. For comparison – in Elaphrosaurus, when scaled to the same lenght of ischia, that part is almost twice as anteroposteriorly short as in Deltadromeus.
All of this strongly suggests that Sereno et al. (1996) and Ibrahim et al. (2020) were right – the elements originally identified as pubes really are pubes.
Most importantly, this removes the only meaningful similarity between Deltadromeus and Bahariasaurus – the distal ischia – and instead highlights yet another major difference: the morphology of the pubes.
I also found this abstract where Deltadromeus agilis is stated to be distinct from any specimen from the Bahariya Formation, so there is nothing left to do but wait.
And I made this about what is and what isn’t Bahariasaurus ingens and its validity:
Some authors have restricted Bahariasaurus solely to a single specimen (IPHG 1922 X47), arguing that no other individuals can be confidently referred to it (Cau, 2008 online; Cau, 2024 pers. comm.), thereby implying potential chimerism of the material. Other researchers have questioned the diagnosability of the material altogether. Chure (2000) stated that Bahariasaurus allegedly lacks a diagnosis and therefore treated it as a nomen nudum. Rauhut (2003) argued that the illustrations in Stromer (1934) do not permit the recognition of diagnostic features and considered the taxon a nomen dubium. Ibrahim et al. (2020) also treated Bahariasaurus as a dubious taxon, though without providing further elaboration.
Here, in contrast to all of the above, I argue that Bahariasaurus ingens is a valid and diagnosable theropod species that includes more than one individual.
Stromer designated the following as type material: IPHG 1922 X47, comprising three incomplete posterior dorsal vertebrae, a fragmentary dorsal rib, the first three sacral centra, pubes, and the proximal part of the ischium; IPHG 1922 X48, consisting of two incomplete posterior dorsal vertebrae and a partial cervical vertebra; and IPHG 1922 X48B, consisting of pubes. Stromer regarded these three specimens as “securely belonging to one species.” This statement is fully justified by the descriptions he provided.
The specimens IPHG 1922 X47 and X48 share posterior dorsal vertebrae. In both, these elements are represented by platycoelous, markedly elongate centra that are strongly constricted at mid-length. In addition, both possess lateral foramina, and the diapophysis is connected to the centrum only via a posterior centrodiapophyseal lamina, with no anterior lamina (Stromer, 1934, pp. 24, 26–27).
The specimen IPHG 1922 X48B, like IPHG 1922 X47, includes a pubis with a straight, slender shaft, a long symphysis, and a flared distal end, bearing a relatively small pubic boot (Stromer, 1934, p. 38). Therefore, IPHG 1922 X47, X48, and X48B - the specimens designated by Stromer as the type material - undoubtedly belong to the same taxon and thus represent syntypes of Bahariasaurus ingens.
In addition to the syntypes, Stromer referred several other specimens to Bahariasaurus with varying degrees of confidence. Some of these can be assigned to B. ingens based on overlapping elements with the syntypes. Most notably, IPHG 1912 VIII 62 includes a partial ilium, proximal pubis, three incomplete posterior dorsal vertebrae, a “caudal” neural arch [here reinterpreted as a possible first dorsal neural arch following Mortimer (online) and Cau & Paterna (2025)], a proximal caudal centrum, and a middle caudal vertebra. Based on its description and illustrations, this individual unquestionably belongs to the same taxon. The proximal right pubis is illustrated (pl. II, fig. 15a, b), and is identical to the corresponding element in IPHG 1922 X48 B (pl. II, fig. 1a, b) in terms of ischial and iliac peduncles, the slender shaft, and absence of an obturator foramen.
One of the posterior dorsal vertebrae is also illustrated (pl. II, fig. 24a, b), and its morphology is nearly identical to the elements in IPHG 1922 X47 and X48 (pl. II, fig. 14a, b). It shares an elongate centrum with similar width and height, a lateral foramen, a low neural arch, and a diapophysis supported ventrally only by a posterior centrodiapophyseal lamina (Stromer, 1934, pp. 27–28). Additionally, the anterior caudal centrum (pl. II, fig. 25), with a lateral foramen and ventral groove (Stromer, 1934, p. 28), corresponds closely to the sacral centra in IPHG 1922 X47 (pl. II, fig. 8, p. 24), further confirming the referral of this specimen to the same species as the syntypes.
Other assignable specimens include the isolated left proximal ischia IPHG 1912 VIII 74 (pl. II, fig. 12) and IPHG 1911 XII 23 (pl. II, fig. 7). Both elements closely resemble the ischium in IPHG 1922 X47 (pl. II, fig. 10) in the morphology of the iliac and pubic peduncles, their size and orientation, and the shallow acetabular margin. The only notable difference is that IPHG 1911 XII 23 is significantly smaller and likely belonged to a juvenile individual.
The same applies to IPHG 1912 VIII 83, which includes two middle caudal vertebrae, one of which is illustrated (pl. II, fig. 11a). Aside from the smaller size, it is very similar to the middle caudal vertebra in IPHG 1912 VIII 62 (pl. II, fig. 26a, b), sharing the same centrum proportions, the position and development of the transverse processes, relatively short prezygapophyses, and a low-edged neural spine (Stromer, 1934, pp. 29, 34–35, 38). This specimen with no doubt represents a juvenile specimen of Bahariasaurus.
Another specimen, IPHG 1912 VIII 60, includes a cranial fragment, scapulocoracoid, vertebral centrum, and eight incomplete caudal vertebrae. Six represent the anterior to middle caudal region, and two are distal caudals. One of the more proximal caudals is illustrated (pl. II, fig. 17a, b). Their morphology is consistent with that of Bahariasaurus (e.g., IPHG 1912 VIII 62), possessing a moderately tall, elongate centrum, a lateral foramen, and a ventral groove - features unique among Bahariya theropods (Stromer, 1934, pp. 31–32).
Finally, IPHG 1912 VIII 82, originally described as a pubis of an indeterminate theropod, was recently reinterpreted as an ischium and referred to Bahariasaurus by Cau & Paterna (2025). I follow this interpretation based on the transversely narrow boot and presence of an obturator process. I also accept the referral to Bahariasaurus. IPHG 1912 VIII 82 (pl. II, fig. 2a–c) shares with other Bahariasaurus ischia (pl. II, figs. 7, 10, 12) the morphology of the iliac peduncle, which is poorly expanded dorsoventrally, a shallow acetabular margin, similar pubic peduncle length and orientation, a flat to slightly concave posterior shaft margin, and the absence of a distinct notch distal to the obturator process. Thus, this specimen likely represents a nearly complete ischium of Bahariasaurus ingens.
Stromer also referred IPHG 1912 VIII 81, consisting of nearly complete pubes approximately half the size of those in IPHG 1922 X47 (Stromer, 1934, p. 35). Chure (2000) questioned this referral based on the sinusoidal curvature of the shaft in IPHG 1912 VIII 81 (pl. II, fig. 3a, b) versus the straight shaft in Bahariasaurus (pl. II, fig. 1a). However, the curvature may result from taphonomic distortion. Still, I follow Mortimer (online) in considering the referral problematic. While the specimen is generally similar in its proximal morphology (iliac and ischiadic peduncles, absence of obturator foramen, long symphysis with interpubic fenestra, small pubic boot), a key difference lies in the fact that the pubes are fully fused distally - unlike the flared distal ends in Bahariasaurus (pl. II, fig. 4a). This fusion is ontogenetically problematic, as older individuals typically exhibit more complete fusion. Therefore, I do not consider this specimen referable to Bahariasaurus, though it cannot be completely ruled out - the fusion might reflect an unusual ontogenetic pattern or pathology.
Stromer also tentatively referred a femur (IPHG 1912 VIII 69) and a fibula (IPHG 1912 VIII 70), both from the same layer as IPHG 1912 VIII 62, to Bahariasaurus. Based on their size and differences from other Bahariya theropods, he proposed their referral to B. ingens (Stromer, 1934, pp. 35–37). However, this cannot be confirmed, as neither the syntypes nor any of the securely referred specimens preserve hindlimb elements. Notably, both IPHG 1912 VIII 69 and 70 were later referred to the Moroccan taxon Deltadromeus agilis by Sereno et al. (1996) and Ibrahim et al. (2020), which is also dubious.
Subsequent authors referred mostly isolated elements from outside the Bahariya Formation to Bahariasaurus. De Lapparent (1960) assigned (under the incorrect name “Baharijasaurus ingens”) one proximal and five middle caudal vertebrae from different individuals, likely from the Farak Formation of Niger. Later, Medeiros and Schultz (2001a, 2002) and Medeiros et al. (2007) compared an isolated ?caudal vertebral centrum from the Alcântara Formation (Brazil) to de Lapparent’s specimens and referred it as “cf. Baharijasaurus”, later “cf. Baharijasaurus ingens”. However, I follow Candeiro et al. (2011) in interpreting both the Brazilian centrum and at least one of the Niger specimens (de Lapparent, pl. V, fig. 4) as quite different from the caudal vertebrae of Bahariasaurus, mainly due to their disproportionately large pleurocoels. They are therefore not referable to Bahariasaurus, and in fact resemble dorsal or sacral vertebrae of juvenile sauropods. The same applies to the vertebra illustrated in pl. VI, fig. 7.
Other de Lapparent vertebrae (pl. V, figs. 16, 17) also differ significantly from Bahariasaurus, lacking pleurocoels and showing strongly ventrally positioned and medially expanded chevron facets. These cannot be referred to B. ingens, and their theropod affinity is also doubtful. Thus, there is no evidence supporting the presence of Bahariasaurus ingens or a close relative in Brazil or Niger. Rauhut (1995) reported “cf. Bahariasaurus” from the Wadi Milk Formation in Sudan, likely referring to caudal vertebrae he described four years later as Carcharodontosauridae indet. (Rauhut, 1999), where he then placed Bahariasaurus. The specimens Vb-607, Vb-717, and Vb-870 differ significantly in centrum proportions and possess small pneumatic foramina within larger depressions, unlike the centrally placed pleurocoels within relatively small depressions in Bahariasaurus. However, one specimen - Vb-871, shows notable similarities, including large internal pneumatic cavities and the anterior articular surface offset above the posterior one, which recalls Tameryraptor but especially Bahariasaurus (Rauhut, 1999; Stromer, 1934).
Vb-871 differs from Tameryraptor and closely resembles Bahariasaurus in having a distinct ventral groove and prezygodiapophyseal lamina (Rauhut, 1999; Stromer, 1934, pp. 31–32). I follow Rauhut’s (1999) interpretation that Vb-871 represents a middle caudal vertebra and is most similar to those of Bahariasaurus (pl. II, fig. 26a, b). However, given its better-developed neural spine, presence of a prezygodiapophyseal lamina, and prominent ventral groove, it was likely more anterior. Although such features are known in more proximal caudals of Bahariasaurus, the available descriptions and illustrations (Stromer, 1934, pp. 31–32; pl. II, fig. 17a, b) suggest that the more pronounced transverse process, more ventrally offset posterior articular surface, and better-developed ventral groove may represent species-level differences. Therefore, I refrain from referring this element to Bahariasaurus ingens, due to low skeletal overlap and some notable morphological differences. However, Vb-871 may represent a form closely related to the Egyptian taxon.
Conclusion:
There is no convincing evidence that Bahariasaurus ingens occurred outside the Bahariya Formation or beyond the specific individuals described by Stromer, listed here for clarity: IPHG 1922 X47, X48, X48B; IPHG 1912 VIII 62, 60, 74, 82, 83; and IPHG 1911 XII 23. Contrary to earlier claims (Rauhut, 1999; Chure, 2000; Rauhut, 2003; Ibrahim et al., 2020), all of these specimens, including the type material alone, allow for recognition of diagnostic features, and thus support the validity of the taxon.Proposed diagnosis
Bahariasaurus ingens is diagnosed by the following unique combination of features:-Sacral centra twice as long as tall, bearing a lateral foramen and a ventral groove;
-Pubes distally flared, with a small pubic boot, presence of an interpubic fenestra, and absence of an obturator foramen;
-Scapulocoracoid with a glenoid whose dorsoventral height exceeds twice the minimum width of the scapular shaft;
-Posterior dorsal vertebrae with elongate centra (more than 1.5 times longer than high anteriorly), bearing lateral foramina; the diapophysis connects to the centrum only posteriorly, lacking an anterior centrodiapophyseal lamina;
-Ischium with a proximally placed, triangular obturator process lacking a distinct notch, and an anteroposteriorly expanded ischial boot.
Bibliography:
Apesteguía, S., Rodríguez, N. D., & Porfiri, P. J. (2016). An unusual new theropod with a didactyl manus from the Upper Cretaceous of Patagonia, Argentina.
Aranciaga Rolando, A. M., Brissón Egli, F., Sales, M., Martinelli, A. G., Canale, J. I., & Ezcurra, M. D. (2018). A supposed Gondwanan oviraptorosaur from the Albian of Brazil represents the oldest South American megaraptoran.
Aranciaga Rolando, A. M., Garcia Marsà, J., & Novas, F. E. (2020). Histology and pneumaticity of Aoniraptor libertatem (Dinosauria, Theropoda), an enigmatic mid-sized megaraptoran from Patagonia.
Aranciaga Rolando, A. M., Méndez, A., Canale, J. I., & Novas, F. E. (2021). Osteology of Aerosteon riocoloradensis (Sereno et al., 2008), a large megaraptoran (Dinosauria: Theropoda) from the Upper Cretaceous of Argentina.
Aranciaga Rolando, A. M., Motta, M. J., Agnolín, F. L., Manabe, M., Tsuihiji, T., & Novas, F. E. (2022). A large Megaraptoridae (Theropoda: Coelurosauria) from Upper Cretaceous (Maastrichtian) of Patagonia, Argentina.
Azuma, Y., & Currie, P. J. (2000). A new carnosaur (Dinosauria: Theropoda) from the Lower Cretaceous of Japan.
Benson, R. B. J., Rich, T. H., Vickers-Rich, P., & Hall, M. (2012). Theropod fauna from southern Australia indicates high polar diversity and climate-driven dinosaur provinciality.
Brusatte, S. L., Benson, R. B. J., & Hutt, S. (2008). The osteology of Neovenator salerii (Dinosauria: Theropoda) from the Wealden Group (Barremian) of the Isle of Wight.
Calvo, J. O., Porfiri, J. D., Veralli, C. D., Novas, F. E., & Poblete, F. (2004). Phylogenetic status of Megaraptor namunhuaiquii Novas based on a new specimen from Neuquén, Patagonia, Argentina.
Cau, A. (2024). A unified framework for predatory dinosaur macroevolution.
Cau, A., & Paterna, A. (2025). Beyond the Stromer's riddle: The impact of lumping and splitting hypotheses on the systematics of the giant predatory dinosaurs from northern Africa.
Coria, R. A., & Currie, P. J. (2016). A new megaraptoran dinosaur (Dinosauria, Theropoda, Megaraptoridae) from the Late Cretaceous of Patagonia.
Evans, D. C., Barrett, P. M., Brink, K. S., & Carrano, M. T. (2015). Osteology and bone microstructure of new, small theropod dinosaur material from the early Late Cretaceous of Morocco.
de Souza, C. A., Soares, M. B., Weinschutz, D. K., Wilner, E., Lopes, R. T., de Araujo, D. S., & Kellner, A. W. A. (2021). The first edentulous ceratosaur from South America.
Frey, E., & Martill, D. M. (1995). A possible oviraptorosaurid theropod from the Santana Formation (Lower Cretaceous, Albian?) of Brazil.
Gomes da Costa Pereira, P. V., Ribeiro, T. B., Brusatte, S. L., Dos Anjos Candeiro, C. R., da Silva Marinho, T., & Paglarelli Bergqvist, L. (2020). Theropod (Dinosauria) diversity from the Potiguar basin (Early-late Cretaceous Albian–Cenomanian), Northeast Brazil.
Ibrahim, N., Sereno, P. C., Varricchio, D. J., Martill, D. M., Dutheil, D. B., Unwin, D. W., Baidder, L., Larsson, H. C. F., Zouhri, S., & Kaoukaya, A. (2020). Geology and paleontology of the Upper Cretaceous Kem Kem Group of eastern Morocco.
Juárez-Valieri, R. D., Porfiri, J. D., & Calvo, J. O. (2011). New information on Ekrixinatosaurus novasi Calvo et al., 2004, a giant and massively-constructed abelisauroid from the Middle Cretaceous of Patagonia.
Kellermann, M., Cuesta, E., & Rauhut, O. W. M. (2025). Re-evaluation of the Bahariya Formation carcharodontosaurid (Dinosauria: Theropoda) and its implications for allosauroid phylogeny.
Kotevski, J., Duncan, R. J., Ziegler, T., Bevitt, J. J., Vickers-Rich, P., Rich, T. H., Evans, A. R., & Poropat, S. F. (2025). Evolutionary and paleobiogeographic implications of new carcharodontosaurian, megaraptorid, and unenlagiine theropod remains from the upper Lower Cretaceous of Victoria, southeast Australia.
Loewen, M. A., Irmis, R. B., Sertich, J. J. W., Currie, P. J., & Sampson, S. D. (2013). Tyrant dinosaur evolution tracks the rise and fall of Late Cretaceous oceans.
Mortimer, M. (n.d.). Theropod Database. https://theropoddatabase.github.io/
Motta, M. J., Aranciaga Rolando, A. M., Rozadilla, S., Agnolín, F. E., Chimento, N. R., Egli, F. B., & Novas, F. E. (2016). New theropod fauna from the Upper Cretaceous (Huincul Formation) of northwestern Patagonia, Argentina.
O'Connor, P. M., Gottfried, M. D., Stevens, N. J., Roberts, E. M., Ngasala, S., Kapilima, S., & Chami, R. C. (2006). A new vertebrate fauna from the Cretaceous Red Sandstone Group, Rukwa Rift Basin, southwestern Tanzania.
Porfiri, J. D., Novas, F. E., Calvo, J. O., Agnolín, F. L., Ezcurra, M. D., & Cerda, I. A. (2014). Juvenile specimen of Megaraptor (Dinosauria, Theropoda) sheds light about tyrannosauroid radiation.
Poropat, S. F., White, M. A., Vickers-Rich, P., & Rich, T. H. (2019). New megaraptorid (Dinosauria: Theropoda) remains from the Lower Cretaceous Eumeralla Formation of Cape Otway, Victoria, Australia.
Rauhut, O. W. M., & Carrano, M. T. (2016). The theropod dinosaur Elaphrosaurus bambergi Janensch, 1920, from the Late Jurassic of Tendaguru, Tanzania.
Rauhut, O. W. M., et al. (2024). A new theropod dinosaur from the Callovian Balabansai Formation of Kyrgyzstan.
Samathi, C., & Sander, P. M. (2019). Two new basal coelurosaurian theropod dinosaurs from the Lower Cretaceous Sao Khua Formation of Thailand.
Sereno, P. C., Dutheil, D. B., Iarochene, M., Larsson, H. C., Lyon, G. H., Magwene, P. M., Sidor, C. A., Varricchio, D. J., & Wilson, J. A. (1996). Predatory dinosaurs from the Sahara and Late Cretaceous faunal differentiation.
Stromer, E. (1934). Ergebnisse der Forschungsreisen Prof. E. Stromers in den Wüsten Ägyptens. II. Wirbeltierreste der Baharije-Stufe (unterstes Cenoman).
Andrea Cau, https://theropoda.blogspot.com/
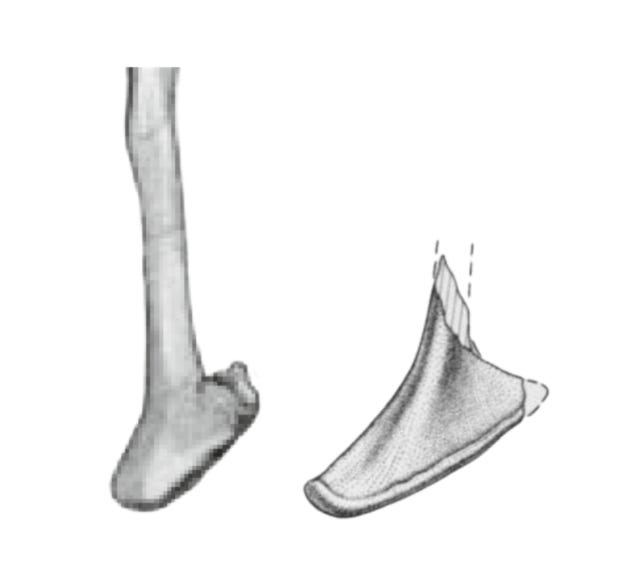
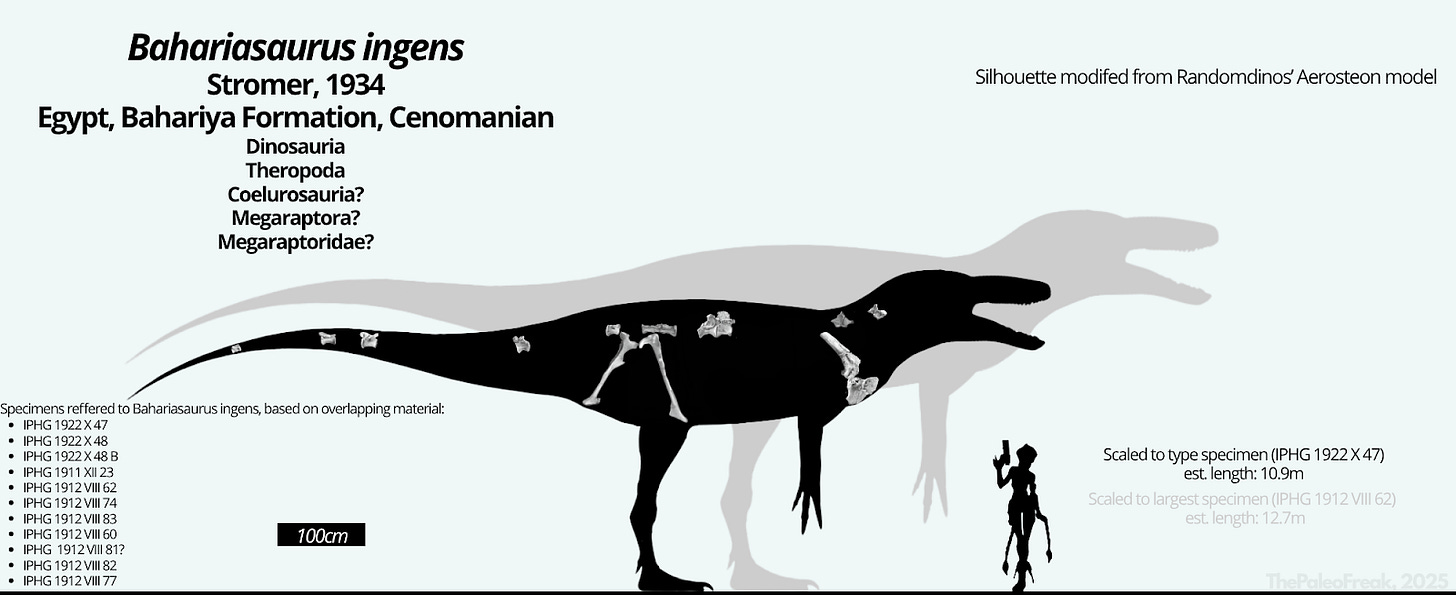


Great post, man! This read like a scientific paper.
How many steps are needed to make Bahariasaurus a carcharodontosaurine (Rauhut, 1995) or Deltadromeus a coelurosaur (Rauhut & Carrano, 2016)?